Matter reflects more or less light waves and resulting colors, which is why we can best see our environment during the day. What we see is basically the reflection of light over a of a variety of elements or atoms that can be, more or less found on earth.
Hydrogen, oxygen, carbon or else gold are common examples of those so-called Atoms.
Each atom possesses specific and singular properties and can widely interact with other specific atoms under specific environmental conditions.
Let us detail a few notable properties of matter that surrounds us and that we are all made of:
Various States
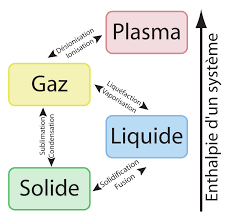

There are hundreds of different atoms and these can be observed on earth in various states: solid, liauids or gas and even other intermediate states such as plasma.
Each element has a stable state on earth depending on the temperature and the atmospheric pressure.
Various Properties
Each element has its own behavior regarding its environment and its neighboring Atoms, and physics and chemistry are the sciences that enable to understand their behavior.
Thus human have tried to make use of materials depending on their advantages and drawbacks their will have for a given application.
For example, paper has been used for 500 years because it is light, easy to produce, and solid and durable enough to transmit and record the information in books or newspapers.
Silicium is widely used to produce micro-electronic components such as transistors because it has specific electrical properties, but also for other reasons such as because it is widely available on earth and can easily be transformed to very very very tiny communication devices.
Various Usage
Humans have developed technologies and know-how to produce matter based on a single element or alloys of complimentary elements.
For example, steel is a composite of Iron and Carbon, which make a very solid material compared to iron or carbon).
A large variety of fabrication processes exist to cleverly combine these elements or compisites to build up functional structures such as concrete structures reinforced with steel bars.
For instance 3D printers are interesting conception devices enabling to produce slowly but in series solid 3D objects using a computer and a special computer driven machine than most often melts a polymer and deposits layers that quickly harden and enable a structure to be build up vertically layers after layers.
But atoms also possess more complex and less visible properties that have been widely used to develop electric and electronic technologies.
Source: Techjango OrdBot 3D Printer.
Mastering this knowledge enables to build electronic devices that are made of various functional parts, layers and components)
Electro-Magnetism
Electric and magnetic properties describe the behavior of atoms or molecules under an electromagnetic field.
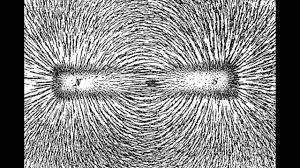
For instance, most metals convey very well electricity, but only some of them like Iron are magnetic. Other atoms, also known as insulators are very weakly sensitive to an electrical field and mostly neither to magnetic fields.
These properties are related to the electric charges contained in atoms and to the capacity of their electrons to move freely around atoms' nucleus.
Infinite possibilities
Humans constantly develop new combinations of materials so as to create better solutions in terms of efficiency, durability or production costs so as to develop more sustainable products.
Thus, If you believe for instance that paper is an insulator and does not conduct the electricity, you will be right most of the case but you will also learn here that an alloys of paper and conductive elements can get conductive enough to be used in electronics.
Is that all I need to know?
Nope! You should also check the basics about atoms which will give you the key to understand what is electronics and what is an electric charge.
This will enable you to understand why some materials are conductors and others are insulators when submitted to an electrical field.